The impact of CH3-GANS on the ph-level of tap water measured inside plasmatic fields created by a Plasma Ball Unit
This article is part of the KF Plasma Times October 2018
Keywords: pH, water, alkalinity, plasma ball unit, PBU.
Published: October 2018.
Laboratory studies were performed to determine whether CH3-GANS of different states of matter in spinning reactors in combination with other GANSes have the potential to increase the pH-value in a glass of tap water inside a dynamic system, called Plasma Ball Unit. As a result, it could be shown, that there is a strong impact of CH3 GANS on the pH-level. Depending on the physical state of the CH3-GANS, an increase in pH-level of up to 1.14 points in 24h could be measured. Several repetitions of the experiment underlined this result.
Introduction
The plasma ball unit (PBU) is a device, invented by Mehran Tavakoli Keshe. The PBU mainly consists of several spheres (balls), which are mounted on motors (dynamic systems) and therefore rotate at a certain speed. Inside the balls, different types of GANSes in different physical states can be placed. The combination of above described dynamic systems creates a “plasmatic field”, which influences the matter inside these fields. In the current study, the influence of the fields of the PBU on tap water is the main focus.
GANS is a new state of matter, discovered by M.T. Keshe. It is the abbreviation for GAs to Nano of Solid. In this state of matter “a molecule of GAs which becomes Nano of itself […] becomes and appears as Solid state of matter” (Keshe, 2012, p. 144). As a result, GANS carries the field spectrum of a gas. This field spectrum affects both “physical material or the property of different matters” (Keshe, 2012, p. 148). The process of producing different types of GANS is well described by the Keshe Foundation and is not part of this paper. We were producing the GANSes as it is described by the Keshe Foundation. In this study, we focus on (1) GANS as precipitate in GANS-water and (2) GANS in a dry state.
Materials and Methods
In this study, we focus on a combination of the GANSes CO2, ZnO and CH3 in the aforementioned, two states of matter. The following experimental setup was introduced:
Experimental constants where (1) the dynamic systems (rotating GANS-balls), filled with CO2 and ZnO precipitate in distilled water, (2) the distance between all dynamic systems used, (3) the rotation speed of all dynamic systems (4.800 rpm), (4) the source of water, (5) the temperature in the room and (6) the starting-time and the time-span of measurement.
The independent variable in the experiment was the dynamic system, filled with CH3 of the following states of matter: (1) CH3 precipitate in distilled water (4ml distilled water and 0,5ml settled precipitate), (2) Dry CH3 powder (2mg) and (3) Dry CH3 powder with an inner ball, filled with high gravitational gold GANS (2ml).
The dependent variable in the experiment was the pH-value of tap water in 4x100ml container, fixed on a carbon fiber holder in different positions. The starting pH-value was 6,6.
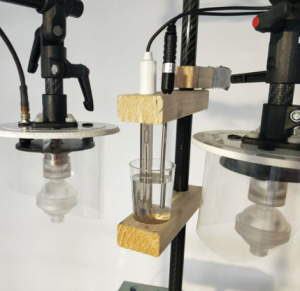
In Figure 1, you can see the experimental setup, the position of the dynamic systems, the position of the water container and the position of the sensors for measuring the pH-value and the temperature of the water.
As one can see in the experimental setup, there is no “physical” contact between the dynamic systems and the water inside the PBU.
As control variable, the holder, including 4 containers of the same tap water, was placed outside the PBU and the pH-level was measured the same way. The control variable is therefore the pH-level of the same tap water but placed outside the PBU.
To sum up the experimental setup, the independent variable (dynamic system with CH3) was changed 3 times and the impact on the depend variable (pH-value of tap water) was measured. Everything else (constants) remained the same. This was controlled by the same tap water outside the PBU.
Measurement procedure
The depend variable was measured by a PCE-PHD Data Logger, applying a pH-electrode PE-03 and a temperature sensor. Before using, the pH-sensor was calibrated, using a certified pH7 calibration liquid:
Figure 2 shows the holder, which places the two sensors inside the water container.
Before a measurement, the PBU was switched off for 45 minutes. During this time, the water containers where filled and the sensors positioned. The experiment started at 2pm every day and lasted 24h.
Results
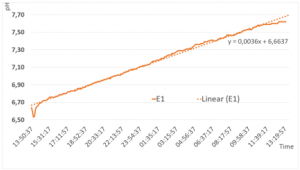
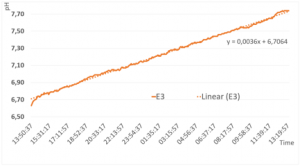
In the first experimental setting, the CH3 system was filled with GANS-water and GANS precipitate. The results from the data-logger, shows a steady increase of the pH-value from 6.6 to 7.62 in the first 24h (Figure 3).
The curve has a slope of 0,0036 The curve seems to flatten at the end, so it can be assumed that the pH-level will increase but with a different slope.
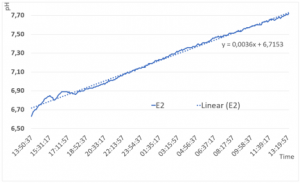
In the second experimental setting, the CH3-System was filled with 5g dry GANS-powder. As a result, the analysis of the data shows, that the maximum pH-level after 24h is 7,72, the slope of the linear trendline is similar to experiment 1.
In Figure 4 the curve does not flatten at the end, therefore we can assume, that the pH-value increases by the same slope after 24h.
The third experimental setting, where an additional gravitational inner ball was placed inside, showed nearly the same results as experiment 2, the maximum pH-level after 24h is 7,74, as depicted in Figure 5.
After 48h, the pH-level was measured again, and it showed a value of 8,30.
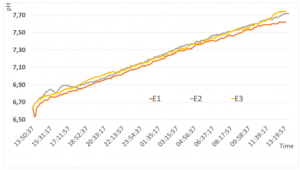
The experiment shows that the pH-level of tap water can be influenced by placing it within plasma fields, created by rotating dynamic systems. Figure 6 shows all three curves of experiment setups 1, 2 and 3 above each other:
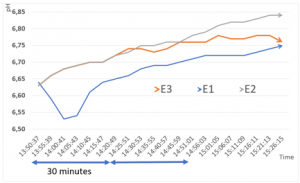
Although all three curves are very similar, the first experimental setting reaches the lowest pH-level and the curve seems to flatten at the end. During the first hours in the PBU, we can observe, that there is a drop in the pH-level of experimental setting 1 in the first 30 minutes. The other settings have similar growth during the same time - as compared in Figure 7.
As there is no physical contact, this influence is attributed to the fields created by the dynamic systems. Therefore, it can be shown, that the fields, created by GANS-reactors, influence matter. Further research will be conducted by changing the different GANS ratios in the dynamic systems.
The human body consists mostly of water. There are different pH-values in different parts of the body. As the plasma fields have no barriers, they will flow through the human body and will, most probably affect the pH-value of the human body too. Further research needs to be completed in this topic.
References
Keshe, M.T. (2012). The Structure of the Light. Second Edition. Stichting the Keshe Foundation: Netherlands.